Fuente:
http://medicalxpress.com/news/2014-09-regenerate-heart-nerve-cells.html
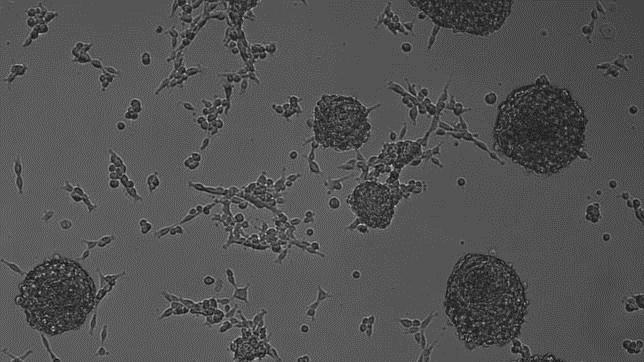
Under a microscope inside a lab at UT Southwestern Medical Center, a dish of cells pulsates with the rhythm of a human heart.
Days ago, these building blocks of heart muscle had a different identity altogether: They were scar-forming cells that proliferate in the wake of a heart attack and weaken the organ's ability to pump blood.
Researchers at UT Southwestern and other institutions may have found a way to reverse the damage that patients sustain from heart attacks, traumatic brain injuries and other conditions.
They accomplish this by converting a less desirable type of cell, such as a scar-forming cell, into a highly desirable one, such as a nerve cell, using a process known as direct reprogramming.
Most recent efforts to convert one cell type into another have relied on stem cells - immature versions of adult cells. But stem cells can be difficult to manipulate and have caused controversy because they are sometimes derived from embryos donated by fertility clinics.
Many scientists say direct reprogramming is a promising new approach to regenerative medicine - a field dedicated to curing disease by helping the body repair and regrow its own tissues.
In recent years, scientists have used the method to grow new brain cells, liver cells, pancreatic tissue and parts of the inner ear responsible for hearing loss.
Researchers hope to one day develop drugs that will enable patients' bodies to repair their own damaged organs, much the way a salamander can regrow its own heart and a python can double the size of its liver.
At UT Southwestern's new Hamon Center for Regenerative Science and Medicine, researchers are focused on cultivating heart and nerve cells.
The center, led by biologist Eric Olson, opened in May with a $10 million gift from the Hamon Charitable Foundation. Its areas of research include stem cells as well as direct reprogramming.
Olson hopes the center will help UT Southwestern attract top college graduates from around the U.S.
"Regenerative biology is one of the most popular majors," he says. "The center will give us a new tool to recruit some of the best students to Dallas."
Olson's career-long interest in how a single cell develops into the trillions that make up the human body led to his lab's current work.
"I wanted to understand how specialized cells were formed and how large sets of genes turned on and off during development," he said.
After pondering what cell type to focus on, Olson settled on muscle cells, because muscles make up 40 percent of our body mass and turn on thousands of genes as they develop.
The idea was that a deep understanding of one particular system could be applied more widely. It could also help scientists gain insight into how diseases develop when errors creep into the process.
Over the years, Olson's lab has uncovered several of the major genes that control muscle development.
Recent work from his lab has led to a promising approach to treating Duchenne muscular dystrophy, a congenital disease that causes muscles to progressively weaken and degenerate.
In the center's inaugural publication this month in the journal Science, Olson and his colleagues outlined a way to "edit" the gene abnormalities responsible for the disorder in a mouse and rectify the body's ability to grow healthy muscle.
Olson's lab extended its work into heart muscle cells in the early 1990s. At the time, Deepak Srivastava, then a postdoctoral researcher, was working in the lab.
"I was trained as a pediatric cardiologist and was interested in how the heart forms in the embryo and how that goes awry in human disease," says Srivastava, who now directs cardiac and stem cell research at the Gladstone Institutes and is a professor at the University of California San Francisco. The two went on to discover many of the genes that control heart development.
Using some of that research, Srivastava pioneered cardiac reprogramming several years later.
"We took the years of knowledge that our lab and other labs had developed about how nature normally makes a heart in the embryo and essentially redeployed those same methods in the adult heart," he says.
In a 2010 paper in the journal Cell, Srivastava showed that scar-forming cells known as fibroblasts could be converted into beating heart cells by adding just three ingredients. Those ingredients, proteins known as transcription factors, are master regulators that flip genes on and off. The genes involved are the same ones that direct heart formation in the womb.
In 2012, Srivastava and Olson, writing separate papers in the same issue of the journal Nature, showed that the process could be performed successfully in living mice.
Each group used a different combination of transcription factors to reprogram the cells, but both found that the technique improved heart function in the animals following a heart attack.
For the last two decades, stem cells have been virtually synonymous with regenerative medicine.
Scientists use them in many different ways. In one approach, researchers take a sample of skin, fat or bone marrow from a patient, convert the mature cells into their previous immature states, then reprogram them to form a new type of tissue.
In a second approach, scientists can extract stem cells from embryos and then convert them.
In clinical trials, researchers have also used a third approach: They have taken so-called adult stem cells from bone marrow and injected them into the heart, hoping the stem cells take on the properties of muscle cells.
Adult stem cells have had mixed results. "The reality is this has shown limited benefits after a decade of clinical trials," says Olson.
So far, researchers have tried direct reprogramming in mice and in human cells in a lab dish.
Chun-Li Zhang, a colleague of Olson's at the Hamon Institute, is using direct reprogramming to rebuild nerve cells, or neurons. He starts with glial cells, which reproduce in the brain and spinal cord following an injury and can form scars.
"Basically, these are the fibroblasts of the brain," he says.
In a series of papers published over the last year, Zhang and his colleagues reported generating new neurons from glial cells in mice following a traumatic brain or spinal cord injury. The lab is now investigating how much the new cells can contribute to the animals' recovery.
Most scientists who work on direct reprogramming are continuing their research on stem cells in parallel. "They each have advantages and disadvantages," says Srivastava.
George Daley, director of stem cell transplantation at Children's Hospital Boston and a past president of the International Society of Stem Cell Research, compared the effectiveness of the two methods in a paper published last month in the journal Cell.
Neurons and heart cells created using direct reprogramming "aren't as close to normal tissue as those generated from stem cells," he said.
Another advantage of using stem cells is that the process creates a lot more cells to work with than direct reprogramming.
"Right now, if you take a dish of fibroblasts in a lab and reprogram that dish, you can get maybe 10 percent of those cells to turn into heart muscle cells, and we'd like to improve that efficiency," says Olson.
Yet direct reprogramming, says Zhang, is more akin to true regeneration. "We design a way to help the tissue repair or regenerate itself," he says. Stem cells, by contrast, involve growing cells in a dish and then injecting them into patients.
The ultimate hope with direct reprogramming is that it will lead to a drug that will stimulate patients' bodies to heal themselves.
With both approaches, Food and Drug Administration-approved treatments are still at least several years away.
Srivastava is testing his direct reprogramming technique in pigs, whose hearts are closer in size to those of humans. The goal is to see if he can generate enough muscle to dramatically improve the heart's pumping ability.
If all goes well, he hopes to approach the FDA with a plan for clinical trials within two to three years.
"It's not inconceivable that we could know if this works in humans within the next five years," he says.